What is Nitric Acid

Nitric acid (HNO3), also known as aqua fortis (Latin for “strong water”) and spirit of niter, is a highly corrosive mineral acid.
The pure compound is colorless, but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as white fuming nitric acid at concentrations above 95%, or red fuming nitric acid at concentrations above 86%.
Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agent.
Structure
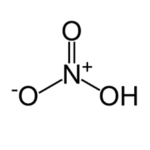
The structure of nitric acid is planar, meaning the chemical structure is flat. There are two major resonance forms of nitric acid. Resonance form occurs when there are multiple ways of drawing the Lewis structure of a compound. A Lewis structure is a diagram that shows how atoms are bonded together and illustrates the presence of non-bonded pairs of electrons in a compound. The double-headed arrow drawn between the two resonance structures is there to show that there is more than one way to draw the structure.
The Uses of Nitric Acid
Nitric acid is also a good oxidizing agent and is used for manufacturing of other inorganic compounds. However, this chemical compound has a lot of other uses. It finds its applications in industries as well as in our everyday life. Let’s look at some of them below.
Industrial Uses
Nitric acid is the building block chemical for the production of many other chemical compounds. It is used in manufacturing several types of polymers like polyamides and polyurethane. Nitric acid is also commonly used as rocket propellants in the aerospace industry. It is also used for manufacturing nitrogen-based compounds like nylon as well as most of the explosives like trinitrotoluene (T.N.T.), nitroglycerin, amongst others. Other uses include, production of nitrate salts, making dyes, coal tar products and drugs. It is also used mostly for the purification of precious metals like platinum, gold, and silver.
Daily Life Uses
The most common use of Nitric acid is found in schools where it is often utilized as a laboratory reagent. Diluted nitric acid is used in woodworks to fabricate maple and pine wood and make them look aged. It is also used in food industries and helps in cleaning food and equipments, etc. More significantly, nitric acid is also used to spot test alkaloids like LSD. Another test known as Colorimetric test which requires nitric acid is used to differentiate between morphine and heroin. These are some of the popular uses of nitric acid. To know more about properties, structure of acids and bases you can keep visiting BYJU’s or download our app for interesting content and learning experience.
Cautions
Nitric acid is a corrosive acid and a powerful oxidizing agent. The major hazard posed by it is chemical burns, as it carries out acid hydrolysis with proeins (amide) and fats (ester), which consequently decomposes living tissue (e.g. skin and flesh). Concentrated nitric acid stains human skin yellow due to its reaction with the keratin. These yellow stains turn orange when neutralized. Systemic effects are unlikely, however, and the substance is not considered a carcinogen or mutagen. The standard first-aid treatment for acid spills on the skin is, as for other corrosive agents, irrigation with large quantities of water. Washing is continued for at least 10–15 minutes to cool the tissue surrounding the acid burn and to prevent secondary damage. Contaminated clothing is removed immediately and the underlying skin washed thoroughly. Being a strong oxidizing agent, nitric acid can react with compounds such as cyanides, carbides, or metallic powders explosively and with many organic compounds, such as turpentine, violently and hypergolically (i.e. self-igniting). Hence, it should be stored away from bases and organics.
PRODUCT IDENTIFICATION
- CAS NO.
- FORMULA
- MOL WT.
- H.S CODE
- SYNONYMS
- 7697-37-2
- HNO3
- 63.02
- 2808.00
- Aqua Fortis
PHYSICAL AND CHEMICAL PROPERTIES
- PHYSICAL STATE
- MELTING POINT
- BOILING POINT
- FREEZING POINT
- SPECIFIC GRAVITY
- SOLUBILITY IN WATER
- DENSITY
- STABILITY
- Liquid
- -41.6°C (-42.9°F)
- 121°C (249.8°F)
- < 0oF
- 1.29
- SOLUBLE
- 1.5192 g / cm3
- The product is stable.
